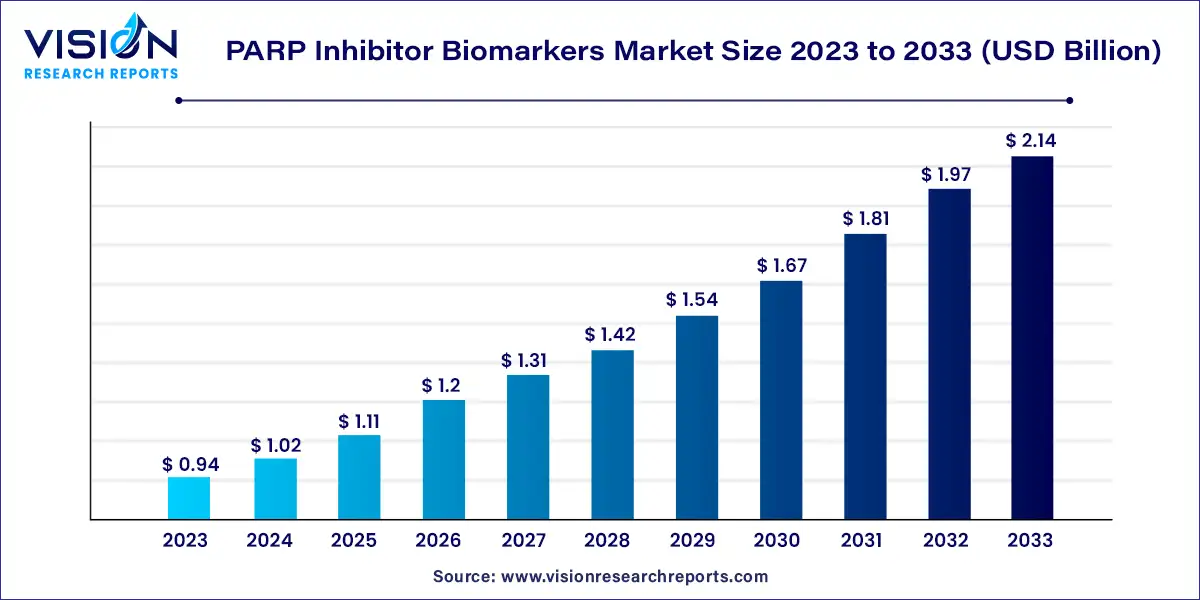
The global PARP inhibitor biomarkers market size was estimated at around USD 0.94 billion in 2023 and it is projected to hit around USD 2.14 billion by 2033, growing at a CAGR of 8.57% from 2024 to 2033.
The field of oncology has witnessed remarkable advancements in recent years, particularly with the emergence of targeted therapies. Among these, PARP (Poly ADP-Ribose Polymerase) inhibitors have garnered significant attention for their potential in cancer treatment. However, the effectiveness of PARP inhibitors varies among individuals, highlighting the importance of biomarkers in personalized medicine.
Introduction to PARP Inhibitor Biomarkers
PARP inhibitors are a class of drugs that inhibit the activity of PARP enzymes, which play a crucial role in repairing damaged DNA in cells. By blocking PARP enzymes, these inhibitors prevent cancer cells from repairing their DNA, leading to their demise. Biomarkers, in this context, refer to measurable indicators that help predict a patient’s response to PARP inhibitor therapy.
Importance of Biomarkers in Cancer Treatment
Personalized medicine aims to tailor treatments to individual patients based on their unique genetic makeup and disease characteristics. Biomarkers serve as invaluable tools in this approach by guiding treatment decisions, predicting treatment outcomes, and minimizing adverse effects. In the context of PARP inhibitors, biomarkers enable clinicians to identify patients who are most likely to benefit from therapy, optimizing treatment efficacy.
Role of PARP Inhibitors in Cancer Therapy
PARP inhibitors have shown promise in the treatment of various cancers, particularly those associated with deficiencies in DNA repair mechanisms, such as BRCA mutations. These inhibitors are used both as monotherapy and in combination with other anticancer agents, offering new therapeutic options for patients with advanced or recurrent cancers.
Get a Sample@ https://www.visionresearchreports.com/report/sample/41243
Understanding PARP Inhibitor Biomarkers
Definition and Types
PARP inhibitor biomarkers encompass a range of genetic and molecular markers that provide insight into a patient’s likelihood of responding to treatment. Key biomarkers include BRCA mutations, homologous recombination deficiency (HRD), and other DNA repair gene alterations.
Mechanism of Action
Biomarkers influence the effectiveness of PARP inhibitors by affecting various aspects of DNA repair pathways. For example, BRCA mutations render cancer cells more susceptible to the cytotoxic effects of PARP inhibition, leading to synthetic lethality.
Significance of Biomarker Identification
Accurate identification of biomarkers is crucial for maximizing the clinical utility of PARP inhibitors. Biomarker testing allows oncologists to stratify patients based on their likelihood of response, facilitating informed treatment decisions and improving patient outcomes.
Current Trends in PARP Inhibitor Biomarkers Market
The PARP inhibitor biomarkers market is witnessing rapid growth, driven by increasing adoption of personalized medicine approaches and advancements in genomic technologies. Key trends include the development of companion diagnostics, expansion of biomarker testing panels, and integration of biomarker data into clinical practice.
Challenges in Biomarker Development
Despite their potential benefits, the development and validation of PARP inhibitor biomarkers present several challenges. These include the need for standardized testing protocols, identification of clinically meaningful biomarkers, and interpretation of complex genomic data.
Future Prospects and Innovations
Ongoing research efforts aim to address existing challenges and further optimize the use of PARP inhibitor biomarkers in clinical practice. Future innovations may include the discovery of novel biomarkers, refinement of predictive algorithms, and integration of biomarker data into electronic health records.
Read More: https://www.heathcareinsights.com/pet-herbal-supplements-market-2/
PARP Inhibitor Biomarkers Market Key Companies
- Myriad Genetics, Inc.
- Ambry Genetics
- Thermo Fisher Scientific, Inc.
- Illumina, Inc.
- CENTOGENE N.V.
- Amoy Diagnostics Co., Ltd.
- Invitae Corporation
- NeoGenomics Laboratories.
- QIAGEN
- Agilent Technologies, Inc.
Recent Developments
- In February 2024, Myriad Genetics, Inc., a company specializing in genetic testing and precision medicine, finalized the acquisition of select assets from Intermountain Health’s Intermountain Precision Genomics (IPG) laboratory business. The acquisition encompasses Precision Oncology Testing, Precision Fluid Testing, and its CLIA-certified laboratory located in St. George, Utah.
- In January 2023, CRISPR technology played a pivotal role in uncovering new markers for resistance to PARP inhibitors in prostate cancer. Scientists utilized genome-wide CRISPR knockout screens to investigate how various genetic mutations affect the effectiveness of PARP inhibitors. A notable finding from these studies is the identification of MMS22L, a gene whose deletion significantly enhances sensitivity to PARP inhibitors.
PARP Inhibitor Biomarkers Market Segmentations:
By Product
- Kits
- Assays
By Services
- BRCA 1 & 2 Testing
- HRD Testing
- HRR Testing
- Others
By Application
- Breast Cancer
- Ovarian Cancer
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41243
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
