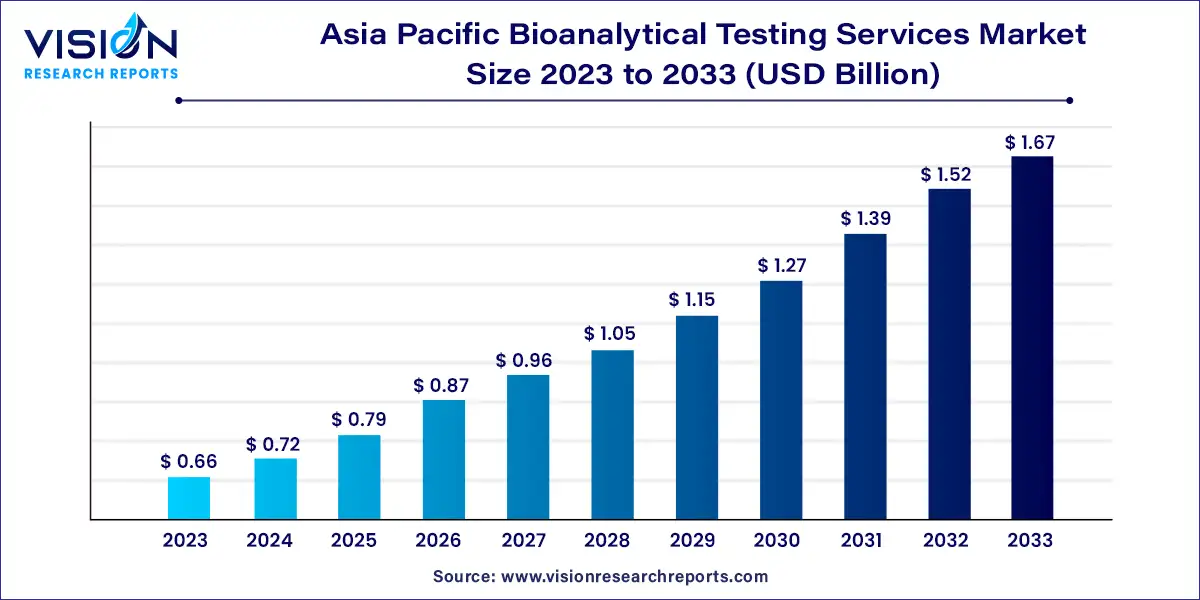
The Asia Pacific bioanalytical testing services market size was estimated at around USD 0.66 billion in 2023 and it is projected to hit around USD 1.67 billion by 2033, growing at a CAGR of 9.75% from 2024 to 2033.
Bioanalytical testing services involve the analysis of biological samples to measure the concentration of drugs, metabolites, or biomarkers. This data is essential for drug development, clinical trials, and personalized medicine. The Asia-Pacific region, with its diverse and expanding economies, offers a unique set of opportunities and challenges for bioanalytical testing services.
Key Highlights:
- Small molecules dominated the market by molecule type, capturing 58% of revenue in 2023.
- Sample analysis was the largest workflow segment, holding a 46% market share in 2023.
- The sample preparation workflow is projected to grow fastest, with a 10.13% CAGR from 2024 to 2033.
- Bioavailability testing held the largest market share by test type in 2023.
- Bioequivalence testing is expected to expand at the fastest rate from 2024 to 2033.
Get Sample@ https://www.visionresearchreports.com/report/sample/41368
Asia Pacific Bioanalytical Testing Services Market Growth Drivers
- Expanding Pharmaceutical and Biotech Industries: Countries like China, India, and Japan are seeing significant growth in their pharmaceutical and biotechnology sectors. This expansion fuels demand for bioanalytical testing services to support drug development and clinical trials.
- Increased Investment in R&D: Governments and private entities in the Asia-Pacific region are investing heavily in research and development, particularly in life sciences and healthcare. This investment drives the need for advanced bioanalytical testing.
- Rising Incidence of Chronic Diseases: The growing prevalence of chronic diseases such as cancer, diabetes, and cardiovascular disorders increases the demand for effective diagnostic and therapeutic solutions, thereby boosting the need for bioanalytical testing.
- Advancements in Technology: Technological innovations, such as high-throughput screening, advanced chromatography, and mass spectrometry, are enhancing the capabilities of bioanalytical testing, making it more precise and efficient.
Asia Pacific Bioanalytical Testing Services Market Key Trends
- Outsourcing to Contract Research Organizations (CROs): Many pharmaceutical and biotech companies are outsourcing their bioanalytical testing to specialized CROs to reduce costs and improve efficiency. This trend is particularly strong in Asia-Pacific due to the region’s cost-effective service offerings.
- Personalized Medicine: With the rise of personalized medicine, there is an increasing demand for bioanalytical testing that can tailor treatments to individual genetic profiles. This trend is driving innovations in bioanalytical methods and technologies.
- Regulatory Changes: The regulatory landscape in the Asia-Pacific region is evolving, with various countries updating their guidelines to align more closely with international standards. This creates both opportunities and challenges for bioanalytical testing providers.
- Emergence of Local Players: Local bioanalytical testing service providers are emerging in the region, offering competitive services and driving innovation. These players are increasingly partnering with international firms to expand their reach and capabilities.
Asia Pacific Bioanalytical Testing Services Market Challenges
- Regulatory Hurdles: Navigating the diverse regulatory environments across Asia-Pacific countries can be complex. Ensuring compliance with local regulations and international standards is a major challenge for bioanalytical testing service providers.
- Quality Assurance: Maintaining high standards of quality and accuracy is crucial for bioanalytical testing. Providers must continually invest in state-of-the-art equipment and skilled personnel to meet the rigorous demands of the industry.
- Data Security: With the increase in data collection and sharing, ensuring the security and confidentiality of sensitive patient data is a significant concern for bioanalytical testing services.
- Competition: The growing number of bioanalytical testing service providers in the region intensifies competition. Companies need to differentiate themselves through superior technology, service quality, and cost-effectiveness.
Asia Pacific Bioanalytical Testing Services Market Future Outlook
The future of the Asia-Pacific bioanalytical testing services market appears promising, with several factors likely to shape its trajectory:
- Continued Growth: The market is expected to continue its upward trend, driven by ongoing investment in healthcare and biotechnology, coupled with rising demand for advanced testing services.
- Technological Advancements: Innovations in bioanalytical technologies will enhance testing capabilities, reduce turnaround times, and improve accuracy. This will enable more efficient drug development and personalized medicine solutions.
- Collaborations and Partnerships: Increased collaborations between local and international firms will foster growth and innovation in the sector. Strategic partnerships will help companies expand their service offerings and geographic reach.
- Regulatory Harmonization: Efforts towards regulatory harmonization across Asia-Pacific countries will simplify compliance and facilitate smoother operations for bioanalytical testing service providers.
Read More@ https://www.heathcareinsights.com/particle-therapy-market-size-and-dynamics/
Asia Pacific Bioanalytical Testing Services Market Top Companies
- Charles River Laboratories
- ICON plc
- Eurofins Scientific
- Syneos Health
- Labcorp Drug Development
- Intertek Group plc
- IQVIA Inc.
- SGS Société Générale de Surveillance SA
- Pharmaron
- Thermo Fisher Scientific Inc. (PPD, Inc.)
Asia Pacific Bioanalytical Testing Services Market Segmentation:
By Molecule
- Small Molecule
- Large Molecule
- LC-MS Studies
- Immunoassays
- PK
- ADA
- Others
- Others
By Test
- ADME
- In-Vivo
- In-Vitro
- PK
- PD
- Bioavailability
- Bioequivalence
- Others
By Workflow
- Sample Preparation
- Protein Precipitation
- Liquid-Liquid Extraction
- Solid Phase Extraction
- Sample Analysis
- Hyphenated Technique
- Chromatographic Technique
- Electrophoresis
- Ligand Binding Assay
- Mass Spectrometry
- Nuclear Magnetic Resonance
- Other Workflow Processes
By Country
- India
- China
- Japan
- South Korea
- Australia
- Thailand
- Indonesia
- Malaysia
- Singapore
- Taiwan
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41368
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
