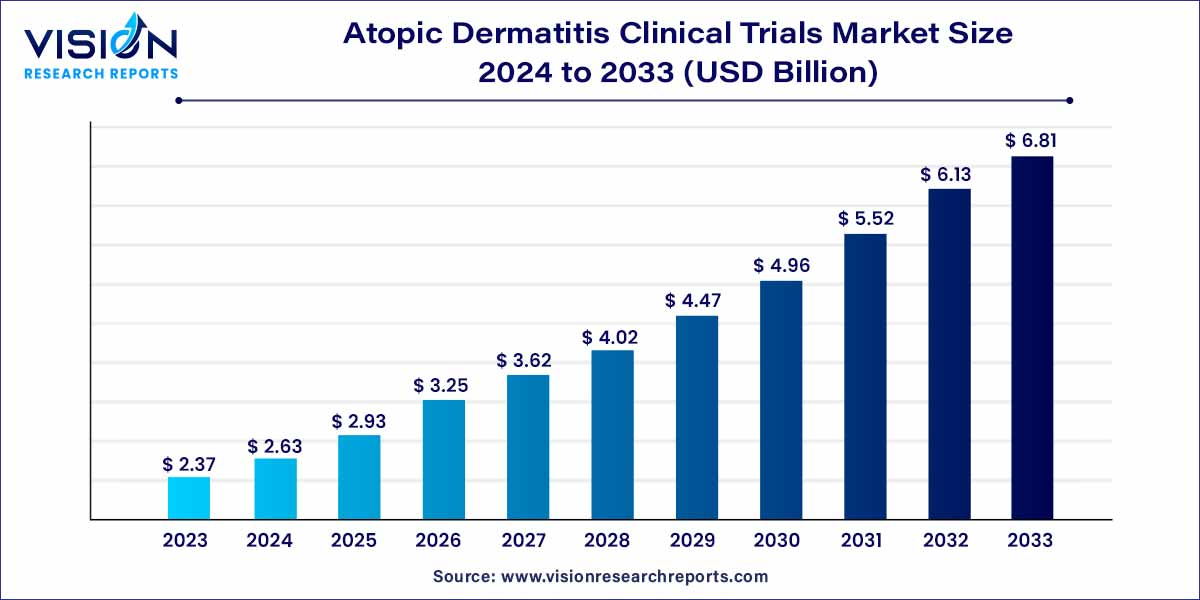
The global atopic dermatitis clinical trials market was estimated at USD 2.37 billion in 2023 and it is expected to surpass around USD 6.81 billion by 2033, poised to grow at a CAGR of 11.14% from 2024 to 2033.
 Key Pointers
Key Pointers
- North America led the market with the largest revenue share 36% in 2023.
- Asia Pacific is predicted to grow at the remarkable CAGR from 2034 to 2033.
- By Molecule, the large molecules led the market with the highest market share of 54% in 2023.
- By Study Designs, the interventional trials accounted for largest market revenue share of 72% in 2023.
- By Phase, the Phase II segment generated the maximum market share of 48% share in 2023.
Atopic Dermatitis Clinical Trials Market Overview
Atopic Dermatitis, commonly known as eczema, affects millions of individuals worldwide, with its characteristic symptoms including itching, redness, and inflammation of the skin. The complexity of this condition has prompted intensive research efforts to discover effective treatments and therapies.
Atopic Dermatitis Clinical Trials Market Growth
The growth of the atopic dermatitis clinical trials market is propelled by several key factors contributing to its expansion. First and foremost, the increasing prevalence of Atopic Dermatitis on a global scale has heightened the urgency for effective therapeutic solutions, stimulating heightened research and development activities. Moreover, advancements in our understanding of the intricate immunological and genetic factors underlying Atopic Dermatitis have paved the way for the exploration of targeted, precision therapies. The growing trend towards personalized medicine has further amplified interest in tailoring treatments to individual patient profiles, fostering a more nuanced and effective approach to Atopic Dermatitis management. Additionally, the robust collaborations between pharmaceutical companies, research institutions, and biotech firms have catalyzed innovation, fostering the discovery and development of novel therapeutic interventions. The emphasis on comparative effectiveness studies not only refines existing treatment protocols but also fuels competition, encouraging the introduction of improved and differentiated therapeutic options. As these factors synergize, the atopic dermatitis clinical trials market is poised for sustained growth, offering promising avenues for enhanced patient care and outcomes.
Get a Sample: https://www.visionresearchreports.com/report/sample/41131
Atopic Dermatitis Clinical Trials Market Trends
- Immunomodulatory Therapies Dominating Research: A prominent trend in the atopic dermatitis clinical trials market is the increasing focus on immunomodulatory therapies. Trials are actively exploring novel drugs that target specific immune pathways implicated in Atopic Dermatitis pathogenesis, aiming for more targeted and efficacious treatments.
- Biologics Revolutionizing Treatment Landscape: The rise of biologics represents a significant trend, with clinical trials investigating the efficacy of monoclonal antibodies and other biopharmaceuticals. These biologics aim to address underlying immune system dysregulation, providing a promising avenue for managing moderate to severe Atopic Dermatitis.
- Personalized Medicine Approaches: Tailoring treatments to individual patient profiles is gaining traction. Clinical trials are increasingly incorporating personalized medicine approaches, considering genetic predispositions, biomarkers, and patient-specific factors to optimize therapeutic outcomes and minimize side effects.
- Topical Innovations for Localized Relief: Research is underway to develop advanced topical formulations that offer targeted relief for localized Atopic Dermatitis symptoms. These formulations may include novel corticosteroids, calcineurin inhibitors, or barrier repair agents, providing more options for managing milder forms of the condition.
- Telemedicine and Remote Monitoring: The integration of telemedicine and remote monitoring in clinical trials is streamlining data collection and patient engagement. This trend not only enhances the efficiency of trials but also reflects the broader shift towards digital healthcare solutions, which may influence the future landscape of Atopic Dermatitis care.
- Focus on Pediatric Atopic Dermatitis: With a rising prevalence of Atopic Dermatitis in children, clinical trials are placing a significant emphasis on pediatric populations. This trend aims to address the unique aspects of pediatric Atopic Dermatitis, ensuring that treatment strategies are safe and effective for this vulnerable patient group.
Molecule Type Insights
Large molecules led the market with the highest market share of 54% in 2023. This high percentage can be attributed to increasing demand for biologics, driven by rising incidence of atopic dermatitis and skin eczema across globe. Several biopharmaceutical companies are expanding their therapeutics pipeline to treat the condition. For instance, as per an article published by Springer Nature Limited in 2022 stated that apart from obtaining regulatory approval for biologics such as anti-IL-13 inhibitor tralokinumab, IL-4Ra inhibitor dupilumab, JAK1/2 inhibitor baricitinib in Europe, there are more than 70 new compounds under development for treatment of mild to severe atopic dermatitis. Hence, increasing pipeline of products under large molecules is primary factor supporting segment’s robust shares and lucrative growth rate across the forecast period.
The small molecules segment is anticipated to witness a stable CAGR during the analysis timeframe. Small molecule drugs have potential to manage atopic dermatitis effectively due to their availability in several dosage forms. For instance, topical form of drugs is much easier to apply and hence has a higher demand across the patient population. Moreover, several pharmaceutical companies are investigating potential of such therapies, and so far, positive results have been witnessed across these developments. For instance, in 2022, Arcutis Biotherapeutics, Inc. announced topline positive results of phase III trials of topical roflumilast cream, a small molecule inhibitor of phosphodiesterase-4 (PDE4) in patients with atopic dermatitis. The drug is under NDA submission review and is expected to gain approval soon.
Study Design Insights
Interventional trials accounted for largest market revenue share of 72% in 2023. High segment shares are majorly due to increasing number of interventional study designs adopted in clinical trials. For instance, as per clinicaltrials.gov in 2022, approximately 60 to 70% of atopic dermatitis clinical trials are interventional studies. Interventional trials have a controlled design, often involving comparison of the intervention group (those receiving treatment) with a control group (those receiving a placebo or standard treatment). Furthermore, increasing number of clinical trials for atopic dermatitis is another considerable factor supporting segment’s growth.
Observational trials segment is anticipated to witness a lucrative growth rate across the analysis period. High growth of segment is majorly due to increasing demand for atopic dermatitis and eczema products globally. Moreover, increasing prevalence of such conditions is one of the primary factors boosting demand for effective therapeutics and augmenting segmental growth. For instance, according to Global Burden of Disease (GBD) estimates, atopic dermatitis, is a chronic skin condition and is widely prevalent and non-communicable. It holds 15th position among non-fatal diseases in terms of disability-adjusted life years, claiming top spot among all skin diseases. The primary impact is notably observed in children.
Phase Insights
Phase II segment had the largest market share of 48% share in 2023. High segment shares are majorly due to increasing number of drugs under phase II trials. Moreover, cost of development of phase II trials is significantly high. Hence, investment across these phases is considerably more, thus supporting high segment shares. Furthermore, several pharmaceutical companies are investing huge amounts of capital in clinical development of these drugs. For instance, in October 2023, Triventi Bio secured USD 92 million in Series A funding to progress its primary antibody program, TRIV-509, into clinical development. Currently, in pre-clinical stage, TRIV-509 is anticipated to be supported by the obtained funding, sustaining operations through Phase IIa clinical trial.
Phase III segment is anticipated to witness a lucrative growth rate across the analysis period. High growth of the segment is majorly due to increasing growing number of pipeline drugs for atopic dermatitis entering phase III phase of development. Moreover, growing partnerships amongst biopharmaceutical companies to expand its clinical pipeline of drugs is another considerable factor supporting segmental growth. In addition, scientific advancements, need for better treatments, a robust developmental pipeline, regulatory requirements, patient-centric approaches, competition in pharmaceutical industry, and collaborative efforts to address the challenges associated with this prevalent skin condition are few of the important factors supporting semental growth.
Regional Insights
North America dominated the market with the largest revenue share 36% in 2023. North America is one of the major contributors to the growth of atopic dermatitis clinical trials industry. This region is home to numerous pharmaceutical and dermatological companies, which are outsourcing part of their development activities to contract service providers, thereby contributing towards the growth of the market.
The U.S. held largest market share in North America. The U.S., has a sizable population, including a significant number of individuals affected by atopic dermatitis. Availability of a large patient pool is attractive for conducting clinical trials, especially those requiring diverse participant groups. The U.S. boasts robust research infrastructure, including well-established clinical trial sites, research institutions, and academic medical centers. This infrastructure facilitates the efficient conduct of clinical trials, making it an attractive region for sponsors and investigators, which are pertinent to gaining competitive advantages.
Asia Pacific is anticipated to witness significant growth in the market. Asia Pacific is the fastest-growing market, as many developed economies are outsourcing clinical trials to countries such as India, China, and South Korea. The evolving business model of outsourcing and R&D activities among key global companies is expected to increase clinical trial services demand in the region, owing to the cost efficiency offered by CROs in countries such as India and China. Furthermore, large & diverse patient pools, recruitment for clinical trials, established clinical infrastructure, and the availability of skilled medical practitioners are supporting market growth.
Read More: https://www.heathcareinsights.com/pulmonary-stents-market/
Key Companies
- Charles River Laboratories
- Imavita
- REPROCELL Inc.
- Oncodesign services
- BIOCYTOGEN
- QIMA LTD
- Novotech
- Redoxis
- Syneos Health
- Hooke Laboratories, LLC
Atopic Dermatitis Clinical Trials Market Segmentations:
By Molecule
- Small molecules
- Large Molecules
By Study Designs
- Interventional
- Observational
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41131
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
