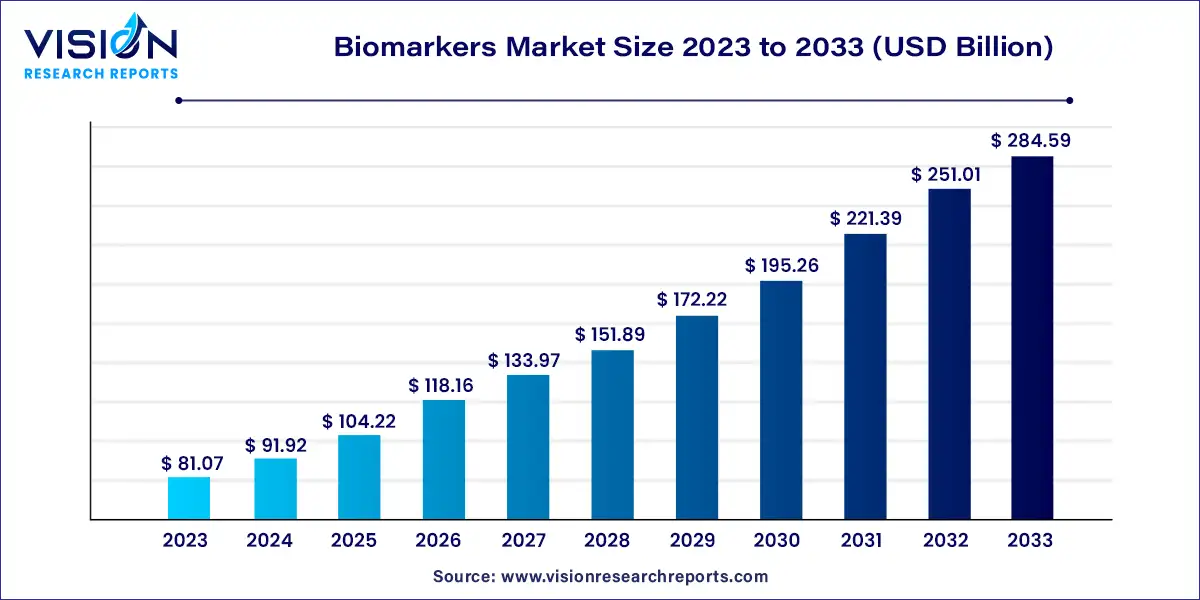
The global biomarkers market size is expected to surpass around USD 284.59 billion by 2033, increasing from USD 81.07 billion in 2023, expanding at a solid CAGR of 13.38% from 2024 to 2033.
The biomarkers market has emerged as a pivotal segment within the healthcare industry, offering immense potential in disease diagnosis, drug discovery, and personalized medicine. Biomarkers are measurable indicators that can reveal the presence of a disease or track the efficacy of therapeutic interventions. These biological markers play a crucial role in identifying diseases early, monitoring disease progression, and tailoring treatments based on individual patient characteristics.
The market for biomarkers is rapidly expanding due to advancements in medical research and technology, particularly in areas like cancer diagnostics, cardiovascular diseases, and neurodegenerative disorders. The ability of biomarkers to provide precise information about a patient’s health status is driving their adoption across clinical settings, leading to better patient outcomes and more targeted therapies.
Biomarkers Market Highlights:
- North America held the largest market share at 44% in 2023.
- Asia Pacific is set to achieve the fastest compound annual growth rate (CAGR) from 2024 to 2033.
- The safety segment accounted for the largest revenue share at 38% in 2023.
- The efficacy biomarkers segment is projected to grow at a significant CAGR from 2024 to 2033.
- The consumables segment emerged as the market leader in 2023.
- The services segment is expected to experience the highest CAGR from 2024 to 2033.
- The drug discovery and development sector generated the largest market share in 2023.
- The diagnostics segment is anticipated to expand at the fastest CAGR from 2024 to 2033.
Get the Sample Pages of Report for More Understanding@ https://www.visionresearchreports.com/report/sample/38602
Top Trends in the Biomarkers Market
- Advancements in Technology:
Technologies like next-generation sequencing (NGS), liquid biopsies, and high-throughput screening are revolutionizing biomarker discovery. NGS allows for rapid and comprehensive analysis of genetic material, enabling the identification of novel biomarkers linked to specific diseases. Liquid biopsies, which analyze blood samples for circulating tumor DNA, offer a less invasive method for early cancer detection and monitoring. - Increased Research and Development:
Investment in R&D is at an all-time high as pharmaceutical and biotech companies recognize the potential of biomarkers in drug development. This includes the discovery of biomarkers that predict patient response to treatments, which can significantly reduce clinical trial costs and accelerate the approval of new therapies. Research is also focusing on biomarkers for chronic diseases, autoimmune disorders, and infectious diseases, expanding the scope of applications. - Regulatory Support:
Regulatory agencies such as the FDA and EMA are increasingly acknowledging the role of biomarkers in drug approval processes. Initiatives like the Biomarker Qualification Program aim to facilitate the development and use of biomarkers in clinical trials, expediting the path to market for new therapies and diagnostics. - Integration of AI and Big Data:
The combination of artificial intelligence (AI) and big data analytics is enhancing biomarker research. Machine learning algorithms can analyze vast datasets from clinical studies and genetic information, identifying patterns and correlations that human researchers might miss. This approach is improving the accuracy of biomarker identification and validation, leading to faster breakthroughs in drug development.
Applications of Biomarkers in Healthcare
Biomarkers have a wide range of applications in healthcare, making them invaluable in both clinical practice and medical research. Some key applications include:
- Diagnostic Biomarkers: These are used to detect the presence of a disease early. For example, biomarkers like PSA (Prostate-Specific Antigen) are used in screening for prostate cancer, while CEA (Carcinoembryonic Antigen) is used for detecting colorectal cancer. Early detection through biomarkers allows for timely intervention and improved patient outcomes.
- Prognostic Biomarkers: These are used to predict the likely course of a disease. In cancer, prognostic biomarkers help clinicians assess how aggressive a tumor is likely to be and guide treatment decisions accordingly.
- Predictive Biomarkers: These indicate how a patient is likely to respond to a particular treatment. This is particularly useful in personalized medicine, where treatments can be tailored based on individual biomarker profiles.
- Monitoring Biomarkers: Used to track the progress of a disease or the effectiveness of a treatment. For example, HIV patients are often monitored using CD4 count and viral load as biomarkers to assess how well they are responding to antiretroviral therapy.
Biomarkers in Drug Discovery and Development
The use of biomarkers in drug discovery and development is transforming the pharmaceutical industry by enabling more targeted and efficient drug development processes. Biomarkers help identify patient populations that are more likely to respond to specific treatments, improving the likelihood of success in clinical trials.
In early-phase clinical trials, biomarkers are used to assess drug safety and efficacy. They provide valuable information about how a drug interacts with biological systems, allowing researchers to make data-driven decisions about whether to proceed to the next phase of development.
In later phases, biomarkers are used to stratify patient populations, ensuring that the right patients are enrolled in clinical trials. This approach not only improves the chances of a drug’s success but also reduces the time and cost associated with clinical trials.
Additionally, biomarkers are being used to develop companion diagnostics, which are tests designed to identify patients who are most likely to benefit from a particular drug. These diagnostics are often used alongside targeted therapies, particularly in oncology, where precision medicine is becoming increasingly important.
Biomarkers in Personalized Medicine
Biomarkers are playing a critical role in the rise of personalized medicine, where treatments are customized based on an individual’s genetic makeup and disease profile. Personalized medicine represents a shift from the traditional “one-size-fits-all” approach to healthcare, allowing for more precise and effective treatments.
For instance, in oncology, the use of biomarkers like HER2 in breast cancer or KRAS in colorectal cancer allows clinicians to determine which patients are likely to benefit from targeted therapies. By identifying specific molecular markers associated with a patient’s tumor, healthcare providers can tailor treatments that are more effective and less likely to cause adverse reactions.
In addition to cancer, biomarkers are being used in other areas of personalized medicine, such as cardiology and neurology. For example, biomarkers like troponin are used in cardiology to assess the risk of heart attacks, while neurodegenerative diseases like Alzheimer’s are being studied with biomarkers like amyloid beta and tau proteins.
The integration of biomarkers with genetic data is also advancing personalized medicine, providing deeper insights into individual health risks and helping to guide preventative healthcare strategies.
Biomarkers Market by Regional Study
North America Market Overview (2023)
- Revenue Share:
- North America dominated the market with a substantial revenue share of 44% in 2023.
- Key Factors Driving Growth:
- High Disease Burden: The region experiences a significant prevalence of various diseases, necessitating the development and use of biomarkers for diagnosis and monitoring.
- Technological Advancements: Continuous innovations in technology facilitate the discovery and application of novel biomarkers in healthcare.
- Increased Consumer Awareness: Growing public knowledge about health and disease management leads to higher demand for diagnostic solutions.
- Supportive Government Initiatives: Government policies and funding programs support research and development in biomarker technologies.
- Improvements in Healthcare Infrastructure: Enhanced healthcare facilities and services contribute to better accessibility and utilization of biomarker testing.
- Local Presence:
- The presence of major market players is expected to enhance the adoption of novel biomarkers, fostering competition and innovation.
- Major Players in the Market:
- Abbott: Known for its extensive portfolio in diagnostics.
- Merck & Co., Inc.: A leader in biopharmaceutical research and development.
- Johnson & Johnson Services, Inc.: Offers a wide range of health solutions including diagnostics.
- Thermo Fisher Scientific, Inc.: Provides innovative diagnostic technologies and services.
- Bio-Rad Laboratories, Inc.: Specializes in developing products for life science research and clinical diagnostics.
- Collaborations:
- There is a growing trend of partnerships among academic institutions, research centers, and key market players to facilitate the development of new biomarkers aimed at diagnosing and monitoring various diseases.
- Focus on Digital Biomarkers:
- An increasing emphasis on developing digital biomarkers is observed, which are expected to revolutionize patient monitoring and data collection.
- Heightened investments in research and collaboration activities are anticipated to significantly drive market growth in this area.
Asia Pacific Market Outlook (2024-2030)
- Growth Rate:
- The Asia Pacific region is projected to achieve the fastest CAGR from 2024 to 2033, indicating a robust market expansion.
- Supporting Factors:
- High Prevalence of Cancer: The rising incidence of cancer in several countries drives demand for innovative diagnostic solutions.
- Increased Funding for Research and Discovery: More investments are being funneled into research initiatives focused on biomarker discovery, enhancing the region’s growth potential.
- Cost-Effective Clinical Trials: Developing nations offer lower costs for conducting clinical trials, making them attractive for research and development activities.
- Rising Research Initiatives: A surge in research initiatives aimed at exploring new diagnostic methods supports overall market growth.
- R&D Advancements:
- Significant research and development activities by biopharmaceutical companies are expected to positively influence the market landscape.
- Example of Advancement:
- In December 2021, Denovo Biopharma LLC announced the discovery of a novel genetic marker that facilitates the development of a gene therapy-based medicine targeting recurrent high-grade glioma.
- This development addresses an urgent unmet medical need, as patients with this condition typically have an estimated survival of less than one year.
Read More@ Digital Biomarkers Market
Biomarkers Market Case Studies
1. Oncology: HER2 Testing in Breast Cancer
Background:
HER2 (human epidermal growth factor receptor 2) is a protein that can promote the growth of cancer cells. In about 15-20% of breast cancers, HER2 is overexpressed, leading to aggressive tumor growth.
Case Study:
Genentech developed trastuzumab (Herceptin), a targeted therapy for HER2-positive breast cancer. Before treatment, patients undergo HER2 testing to determine their receptor status. This biomarker testing ensures that only patients with HER2-positive tumors receive trastuzumab, leading to improved outcomes.
Outcome:
Clinical trials demonstrated that HER2-positive patients receiving trastuzumab had significantly better survival rates compared to those who did not receive the therapy. This case highlights how biomarkers can guide treatment decisions, optimizing patient outcomes and minimizing unnecessary side effects.
2. Cardiology: Troponin Levels in Acute Myocardial Infarction (AMI)
Background:
Troponin is a protein released into the bloodstream when the heart muscle is damaged, making it a key biomarker for diagnosing heart attacks.
Case Study:
Many hospitals adopted high-sensitivity troponin tests to quickly diagnose AMI. In a study conducted in a major urban hospital, clinicians implemented a rapid troponin test protocol for patients presenting with chest pain.
Outcome:
The protocol reduced the time to diagnosis and treatment for AMI patients by up to 60%. Early identification allowed for timely interventions, significantly improving patient outcomes and reducing mortality rates.
3. Infectious Diseases: Biomarkers in Sepsis Diagnosis
Background:
Sepsis is a life-threatening condition triggered by an infection, and its timely diagnosis is crucial for survival.
Case Study:
A research team developed a panel of biomarkers, including procalcitonin (PCT) and C-reactive protein (CRP), to improve sepsis diagnosis. The study was conducted in a large hospital setting where patients suspected of having sepsis were enrolled.
Outcome:
Using the biomarker panel, the time to appropriate antibiotic treatment was significantly reduced. The implementation of this diagnostic approach led to a decrease in hospital mortality rates and improved patient management, demonstrating the value of biomarkers in critical care.
4. Neurology: Alzheimer’s Disease Biomarkers
Background:
Early diagnosis of Alzheimer’s disease is critical for effective management and treatment. Biomarkers in cerebrospinal fluid (CSF) and blood can indicate the presence of amyloid plaques and tau tangles, hallmark features of Alzheimer’s.
Case Study:
A pharmaceutical company, along with research institutions, investigated the use of amyloid-beta and tau biomarkers in patients with mild cognitive impairment (MCI). The study aimed to evaluate the efficacy of a new Alzheimer’s drug based on these biomarkers.
Outcome:
The findings indicated that patients with specific biomarker profiles were more likely to progress from MCI to Alzheimer’s disease. This information not only assisted in patient stratification for clinical trials but also offered insights into early intervention strategies.
Key Companies & Market Share Insights
Prominent players in the market include F. Hoffmann-La Roche AG, Abbott, QIAGEN, and PerkinElmer Incorporated. These companies are prioritizing geographic expansion and securing market approvals for innovative products. They are also making significant investments in advanced technology and infrastructure to efficiently process and analyze large volumes of samples. Additionally, many of these firms are pursuing strategic partnerships with other companies and distributors to enhance their market presence.
Emerging participants in the market include Atlas Genetics Ltd., Hologic, Inc., Myriad Genetics, Inc., and Genomic Health, Inc. These companies are seeking funding from government agencies and healthcare organizations, coupled with the launch of innovative products to explore untapped opportunities.
Key Biomarkers Companies:
- F. Hoffmann-La Roche AG
- Epigenomics AG
- Abbott
- Thermo Fisher Scientific Inc.
- General Electric
- Eurofins Scientific
- Johnson & Johnson Services, Inc.
- QIAGEN
- Bio-Rad Laboratories, Inc.
- Siemens Healthineers AG
- Merck KGaA
- PerkinElmer Inc.
- Agilent Technologies, Inc.
Biomarkers Market Segmentations:
By Type
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
By Product
- Consumable
- Services
- Software
By Application
- Diagnostics
- Drug Discovery & Development
- Personalized Medicine
- Disease Risk Assessment
- Others
By Disease
- Cancer
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Cardiovascular Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Neurological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Immunological Diseases
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarkers
- Prognostics Biomarkers
- Validation Biomarkers
- Others
- Safety Biomarkers
- Efficacy Biomarkers
- Predictive Biomarkers
- Surrogate Biomarkers
- Pharmacodynamic Biomarker
- Prognostics Biomarkers
- Validation Biomarkers
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/38602
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
