The CAR T-cell therapy market size is poised to grow by USD 30.25 billion by 2033 from USD 3.71 billion in 2023, exhibiting a CAGR of 23.35% during the forecast period 2024-2033.
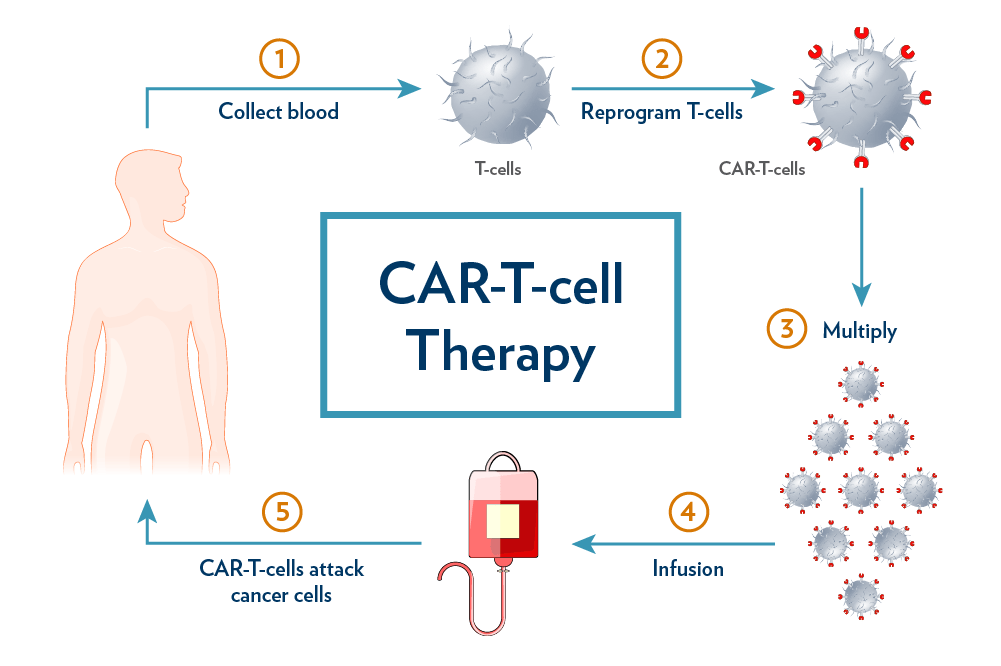
The CAR T-cell therapy market encompasses the biopharmaceutical industry segment that is focused on the development, production, and commercialization of chimeric antigen receptor (CAR) T-cell therapies. Chimeric antigen receptor (CAR) T cells are fusion proteins that direct T cells to antigens on tumor cells, resulting in an antitumor immune response. Over a decade ago, CAR T cells that target CD19, which is expressed in malignant B cells, demonstrated substantial efficiency in clinical studies involving patients with relapsed and refractory B cell malignancies.
These cells produced full response rates of 40-54%, 67%, and 96-74% in patients with aggressive B cell lymphomas, indolent B cell lymphoma, and mantle cell lymphoma. CAR T cell treatments, which have been authorized by the FDA for treating R/R aggressive B cell lymphomas, indolent B cell lymphomas, and mantle cell lymphomas, have emerged as an important component of the therapy landscape for a variety of hematological malignancies.
CAR T-cell Therapy Market Highlights:
- North America led the market with a commanding share of 67% in 2023.
- The Asia-Pacific region is projected to experience significant growth, with an impressive compound annual growth rate (CAGR) of 27.68% from 2024 to 2033.
- Yescarta was the top-performing product, accounting for 44% of the market share in 2023.
- In terms of disease indication, Lymphoma dominated, capturing 58% of the market share in 2023.
- Multiple Myeloma is anticipated to experience the highest CAGR of 22.07% from 2024 to 2033.
- The Hospital segment was the largest end-use category, holding 58% of the market share in 2023.
Get Sample@ https://www.visionresearchreports.com/report/sample/40008
CAR T-Cell Therapy Market Dynamics
Driver: Increasing cases of cancer
Increasing cancer prevalence is a major driver of the CAR T-cell therapy market. Cancer is a major worldwide health concern, accounting for almost one in every six fatalities and one in every four from noncommunicable diseases (NCDs). It is a serious impediment to improving life expectancy, with significant social and financial implications.
In 2022, GLOBOCAN projections showed the worldwide cancer burden, with 36 cancer types and an emphasis on the top ten. The study also looked at regional heterogeneity in 20 selected global areas and estimated the future cancer burden in 2050 using global population estimates. The study also assessed the burden using the human development index IHDI) and predicted the future burden of cancer in 2050.
Restraint: Antigen escape
CAR T-cell therapy poses hurdles when tumors acquire resistance to single antigen-targeting CAR designs. This condition, known as antigen escape, occurs even when malignant cells in many patients exhibit partial to total loss of target antigen expression.
Despite long-lasting responses in relapsed and refractory ALL patients, new follow-up data indicate a shared disease resistance mechanism, including downregulation/loss of CD19 antigen in 30-70% of individuals with recurrent illness following therapy. Strategies for tandem CARs. Preliminary clinical trial outcomes utilizing dual-targeted CAR-T cells have been promising, with greater anti-tumor responses compared to single-target treatment.
Opportunity: New technology for improving therapy efficiency
Multiple myeloma therapy options are limited, although CAR T-cells, modified T cells with lymphocyte-like signaling molecules, have shown promise in targeting genes in malignant cells. Clinical trials are currently being conducted to investigate combinations of medicines, but identifying effective targets and appropriate combinations remains difficult.
To lower the chance of relapse, researchers are working on new antigens and pharmacological treatments and changing the design of CAR T cells. Maintenance therapy and stronger medications in place of traditional chemotherapy regimens can also help enhance treatment outcomes. To advance CAR T-cell therapy, bioengineering, fundamental mechanistic research, and clinical trials are required. CRISPR-Cas9 technology enables genome-wide screening for new genes that can improve CAR T-cell resistance and capacities.
Case Studies of CAR T-cell Therapy Market
1. Kymriah (Novartis) in Acute Lymphoblastic Leukemia (ALL)
Overview:
- Patient Population: Children and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
- Implementation:
- Collection: T cells are collected from the patient through leukapheresis.
- Modification: The T cells are genetically engineered to express the CAR targeting CD19, a protein on the surface of B cells.
- Infusion: The modified T cells are infused back into the patient after a preparative chemotherapy regimen.
Outcome:
- Efficacy: Clinical trials showed a high complete remission rate, with over 80% of patients achieving remission within three months of treatment.
- Safety: The therapy was associated with severe side effects, including CRS and neurotoxicity, but these were managed with supportive care and interventions.
Impact: Kymriah’s approval marked a significant milestone in CAR T-cell therapy, demonstrating its potential as a life-saving treatment for patients with otherwise terminal leukemia.
2. Yescarta (Gilead Sciences) in Large B-Cell Lymphoma (LBCL)
Overview:
- Patient Population: Adults with relapsed or refractory large B-cell lymphoma.
- Implementation:
- Collection: T cells are harvested from the patient.
- Modification: T cells are engineered to express a CAR targeting CD19.
- Infusion: After conditioning chemotherapy, the modified T cells are reinfused into the patient.
Outcome:
- Efficacy: Clinical studies demonstrated a 72% overall response rate with 51% achieving a complete response.
- Safety: Side effects included CRS and neurotoxicity, but these were manageable with existing protocols.
Impact: Yescarta expanded the indications for CAR T-cell therapy and provided a new treatment option for patients with aggressive lymphomas.
3. Breyanzi (Bristol-Myers Squibb) in Large B-Cell Lymphoma (LBCL)
Overview:
- Patient Population: Adults with relapsed or refractory LBCL.
- Implementation:
- Collection: T cells are collected from the patient.
- Modification: The cells are engineered to express a CAR targeting CD19.
- Infusion: After a preparative regimen, the engineered T cells are infused back into the patient.
Outcome:
- Efficacy: Clinical trials reported a 73% overall response rate with 54% of patients achieving a complete response.
- Safety: The therapy was associated with CRS and neurotoxicity, though these adverse effects were typically managed effectively.
Impact: Breyanzi’s approval reinforced the efficacy of CAR T-cell therapy in treating refractory LBCL and contributed to the growing body of evidence supporting CAR T-cell therapy as a viable option for these cancers.
Recent Developments
- In June 2024, A study published in Nature Medicine found that a chimeric antigen receptor (CAR) T cell therapy developed by City of Hope®, a cancer research organization based in the United States, can effectively treat advanced prostate cancer patients with minimal side effects and promising therapeutic activity, despite the challenges associated with treating prostate cancer with immunotherapy.
- In June 2024, research published in The New England Journal of Medicine discovered that second tumors following chimeric antigen receptor (CAR) T-cell treatment are uncommon after investigating the prevalence of second tumors in 724 individuals from a single institution.
- In June 2024, Shanghai First Song Therapeutics is developing Anti-HER2-CAR-T Cells, which are now in Phase I for solid tumor therapy. According to GlobalData, the drug’s PTSR and LoA scores are comparable to the 70% threshold for Phase I solid tumor medicines, showing promise for Phase II trials.
Read More@ https://www.heathcareinsights.com/liquid-biopsy-market/
CAR T-cell Therapy Market Key Companies
- Bristol-Myers Squibb Company
- Novartis AG
- Gilead Sciences, Inc.
- Johnson & Johnson Services, Inc.
- JW Therapeutics (Shanghai) Co., Ltd.
- bluebird bio, Inc.
- Merck & Co., Inc.
- Sangamo Therapeutics
- Sorrento Therapeutics, Inc.
- GSK plc.
CAR T-cell Therapy Market Segmentations:
By Product
- Abecma (idecabtagene vicleucel)
- Breyanzi (lisocabtagene maraleucel)
- Carvykti (ciltacabtagene autoleucel)
- Kymriah (tisagenlecleucel)
- Tecartus (brexucabtagene autoleucel)
- Yescarta (axicabtagene ciloleucel)
- Others
By Disease Indication
- Leukemia
- Lymphoma
- Multiple Myeloma
- Others
By End-Use
- Hospitals
- Cancer Treatment Centers
By Region
- North America
- Europe
- Asia Pacific
- Rest of World
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/40008
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
