The global gene delivery technologies market value stood at USD 4.93 billion in 2023 and it is predicted to rise to USD 14.56 billion by 2033 with growing at a CAGR of 11.44% from 2024 to 2033.
Gene delivery technology involves the methods and tools used to introduce genetic material, such as DNA or RNA, into cells. This process is crucial for gene therapy, which aims to treat or prevent diseases by correcting defective genes or introducing new ones. Common gene delivery methods include viral vectors, non-viral vectors, and physical techniques like electroporation.
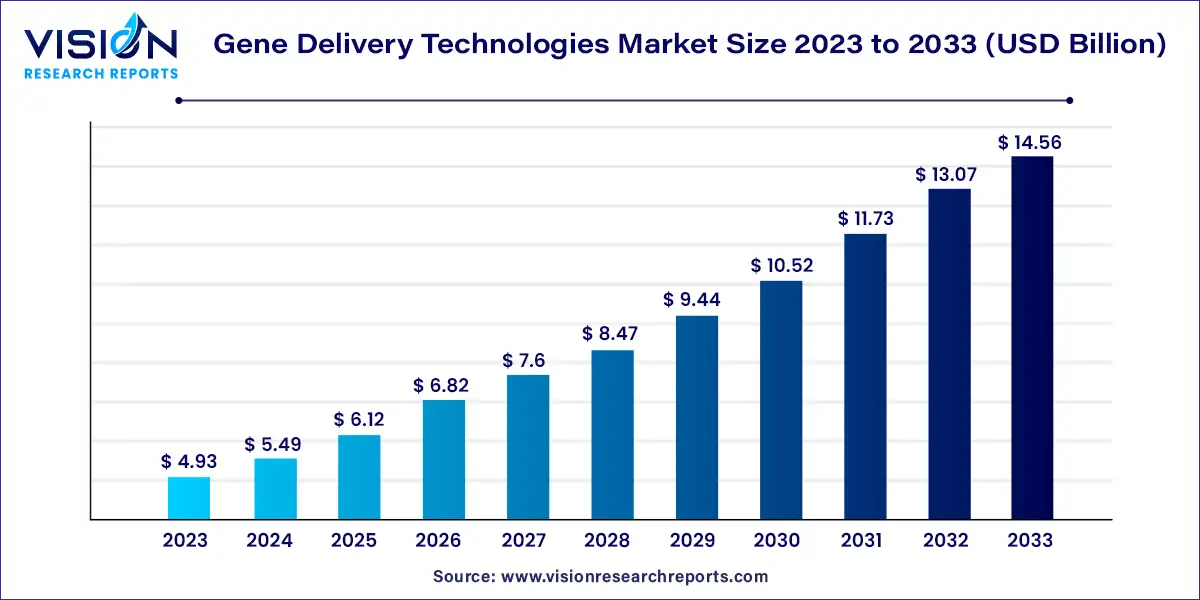
Gene Delivery Technologies Market Highlights:
- North America led the global market, holding the highest market share at 38% in 2023.
- The Asia-Pacific region is anticipated to witness significant growth throughout the forecast period.
- In terms of mode, the biological segment accounted for the largest market share, capturing 60% in 2023.
- The chemical segment, categorized by mode, is expected to show a strong growth rate with a projected compound annual growth rate (CAGR) of 11.93% between 2024 and 2033.
- By application, the gene therapy segment held the largest market share, generating 37% in 2023.
- The ex vivo method emerged as the leading method, securing a market share of 42% in 2023.
- The in vitro method is forecasted to grow at a CAGR of 11.83% from 2024 to 2033.
Why is Gene Delivery Important?
The significance of gene delivery technologies lies in their potential to address the root causes of genetic disorders and provide more targeted treatments. Traditional treatments often focus on managing symptoms rather than curing diseases. In contrast, gene therapy can potentially offer long-term solutions by altering the genetic code within affected cells. This transformative approach has the potential to change how we understand and treat various illnesses, including cancer, cystic fibrosis, and hemophilia.
Get Sample@ https://www.visionresearchreports.com/report/sample/41578
Key Market Drivers
1. Increasing Prevalence of Genetic Disorders
The rising number of genetic disorders worldwide is one of the primary drivers of the gene delivery technologies market. Conditions such as cystic fibrosis, muscular dystrophy, and certain types of cancer are caused by genetic mutations that can potentially be treated through gene therapy. As awareness and diagnosis of these conditions increase, so does the demand for effective treatment options.
2. Advancements in Biotechnology
Rapid advancements in biotechnology, including the development of CRISPR and other gene-editing tools, have significantly improved the precision and efficiency of gene delivery methods. These innovations enable scientists to manipulate genes with higher accuracy, reducing the risk of off-target effects and improving patient outcomes.
Case Studies in Gene Delivery Technologies Market
1. Luxturna: Revolutionizing Treatment for Inherited Retinal Dystrophy
Company: Spark Therapeutics
Technology Used: Adeno-Associated Virus (AAV) Vector
Condition Treated: Inherited Retinal Dystrophy due to RPE65 Gene Mutation
Overview:
Luxturna is one of the pioneering gene therapy treatments, specifically designed to treat a rare form of inherited retinal dystrophy caused by mutations in the RPE65 gene. This condition leads to severe vision impairment and eventually complete blindness. Luxturna delivers a functional copy of the RPE65 gene directly into retinal cells using an adeno-associated virus (AAV) vector. This method allows the retinal cells to produce the normal protein required for vision, thereby improving the patient’s sight.
Outcome:
Clinical trials for Luxturna showed significant improvement in patients’ vision. Treated individuals demonstrated enhanced ability to navigate a low-light obstacle course, a clear sign of the therapy’s effectiveness. In 2017, Luxturna received FDA approval, making it the first gene therapy approved in the United States for an inherited disease. This success not only highlighted the potential of gene delivery technologies but also set the stage for further advancements in treating genetic eye disorders.
2. Zolgensma: A Breakthrough in Spinal Muscular Atrophy Treatment
Company: Novartis
Technology Used: AAV9 Vector
Condition Treated: Spinal Muscular Atrophy (SMA)
Overview:
Zolgensma is a groundbreaking gene therapy developed to treat Spinal Muscular Atrophy (SMA), a genetic disorder characterized by the loss of motor neurons, leading to muscle wasting and early death. The condition is caused by mutations in the SMN1 gene. Zolgensma uses an AAV9 vector to deliver a functional copy of the SMN1 gene into the patient’s motor neurons. Administered as a one-time intravenous infusion, Zolgensma addresses the root cause of SMA by restoring SMN protein production.
Outcome:
Clinical trials demonstrated that Zolgensma significantly improved survival rates, motor function, and overall quality of life in infants diagnosed with SMA. Infants treated with Zolgensma showed the ability to sit, roll, and even walk, milestones rarely reached by those with SMA. Approved by the FDA in 2019, Zolgensma has become a standard of care for SMA, exemplifying the life-changing potential of gene delivery technology.
3. CRISPR-Based Gene Editing for Sickle Cell Disease
Organizations: CRISPR Therapeutics, Vertex Pharmaceuticals
Technology Used: CRISPR-Cas9 Gene Editing
Condition Treated: Sickle Cell Disease
Overview:
Sickle Cell Disease (SCD) is a genetic disorder characterized by abnormally shaped red blood cells, leading to severe pain, anemia, and potential organ damage. A novel approach using CRISPR-Cas9 gene-editing technology was developed to treat SCD by reactivating fetal hemoglobin production. This treatment involves extracting hematopoietic stem cells from the patient’s bone marrow, editing them using CRISPR to disrupt the BCL11A gene, and reintroducing the modified cells back into the patient.
Outcome:
Early clinical trials for this CRISPR-based therapy showed promising results, with patients experiencing a significant reduction in pain crises and independence from regular blood transfusions. One of the first patients treated with this approach remained symptom-free and without blood transfusions for more than a year. These findings underline the potential of gene editing as a transformative treatment for SCD and other complex genetic disorders.
4. Glybera: The First Gene Therapy for Lipoprotein Lipase Deficiency (LPLD)
Company: uniQure
Technology Used: Adeno-Associated Virus (AAV1) Vector
Condition Treated: Lipoprotein Lipase Deficiency (LPLD)
Overview:
Glybera was the first gene therapy approved for treating Lipoprotein Lipase Deficiency, a rare genetic disorder characterized by the inability to break down fats in the blood due to a deficiency in the lipoprotein lipase enzyme. This condition leads to severe pancreatitis and abdominal pain. Glybera uses an AAV1 vector to deliver a copy of the LPL gene into muscle cells, enabling these cells to produce the lipase enzyme and break down fat in the bloodstream.
Outcome:
Glybera’s clinical trials showed that treated patients experienced a reduction in pancreatitis episodes and a decrease in fat levels in their blood. Although Glybera marked a milestone in gene therapy by receiving approval from the European Medicines Agency (EMA) in 2012, it was later withdrawn from the market due to high costs and limited patient population. Despite its market challenges, Glybera’s development underscored the feasibility and impact of gene delivery technologies in treating metabolic disorders.
Challenges Facing the Gene Delivery Technologies Market
1. Safety and Ethical Concerns
Despite its potential, gene therapy raises safety and ethical concerns. The use of viral vectors for gene delivery poses risks of immune reactions and insertional mutagenesis, where the inserted gene disrupts normal cellular functions. Additionally, ethical debates around gene editing and its potential misuse for non-therapeutic purposes, such as designer babies, are ongoing.
2. High Development Costs
The development and commercialization of gene therapies are costly and time-consuming. Clinical trials for gene therapies require significant investment, and the complexity of manufacturing gene delivery systems adds to the cost. These factors can hinder the entry of smaller companies into the market and limit the availability of gene therapies to patients.
Government Initiatives and Support in the Gene Delivery Technologies Market
Government initiatives and support play a critical role in the advancement and commercialization of gene delivery technologies. Recognizing the potential of these technologies to revolutionize healthcare and address unmet medical needs, various governments around the world have introduced policies, funding programs, and regulatory frameworks to promote research, development, and deployment of gene therapies. Below is an overview of key government initiatives and support mechanisms that are shaping the gene delivery technologies market.
1. Funding and Grant Programs
Government funding and grants are vital for the development and commercialization of gene delivery technologies. In many countries, government agencies have established dedicated funding programs to support basic and translational research in gene therapy. These programs provide financial resources to academic institutions, research organizations, and biotech companies working on innovative gene delivery methods.
- United States: The National Institutes of Health (NIH), the largest public funder of biomedical research in the world, allocates substantial funding for gene therapy research through its various institutes, including the National Institute of Neurological Disorders and Stroke (NINDS) and the National Cancer Institute (NCI). Additionally, the U.S. Department of Defense (DoD) has funded gene therapy research to address medical challenges faced by military personnel.
- European Union: The Horizon Europe program, which succeeded the Horizon 2020 program, is the EU’s key funding framework for research and innovation, including gene therapy projects. The European Commission also supports public-private partnerships through the Innovative Medicines Initiative (IMI), which funds collaborative research projects aimed at advancing gene delivery technologies.
- China: The Chinese government has made significant investments in gene therapy research through its National Natural Science Foundation and other state-funded programs. China’s strategic plans, such as the “Made in China 2025” initiative, emphasize the development of biotechnology, including gene therapy, to enhance healthcare outcomes.
2. Regulatory Support and Fast-Track Approvals
Regulatory agencies around the world have recognized the unique nature of gene therapies and have established specific guidelines and pathways to facilitate their approval. These regulatory frameworks aim to ensure the safety and efficacy of gene therapies while expediting the review and approval process.
- U.S. Food and Drug Administration (FDA): The FDA has created a framework for the regulation of gene therapies, including the establishment of the Office of Tissues and Advanced Therapies (OTAT) under the Center for Biologics Evaluation and Research (CBER). The FDA’s breakthrough therapy designation, regenerative medicine advanced therapy (RMAT) designation, and fast-track designation provide accelerated review pathways for gene therapies that demonstrate the potential to treat serious or life-threatening conditions.
- European Medicines Agency (EMA): The EMA’s Committee for Advanced Therapies (CAT) is responsible for assessing gene therapy products in Europe. The EMA offers the Priority Medicines (PRIME) scheme to support the development of therapies that address unmet medical needs, providing scientific advice and accelerated assessment options for promising gene therapies.
- Japan’s Pharmaceuticals and Medical Devices Agency (PMDA): Japan has implemented a regulatory framework specifically for regenerative medicines, including gene therapies. The PMDA’s conditional early approval system allows for the conditional approval of innovative therapies based on preliminary evidence, facilitating earlier access to patients while further data is collected.
Read More@ https://www.heathcareinsights.com/health-and-wellness-market/
Top Companies in Gene Delivery Technologies Market
- Thermo Fisher Scientific Inc.
- Horizon Discovery Ltd.
- QIAGEN
- Oxford Biomedica PLC
- OriGene Technologies, Inc.
- SignaGen Laboratories
- Promega Corporation.
- SIRION BIOTECH GmbH
- Flash Therapeutics
- Takara Bio Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd
- System Biosciences, LLC.
- Catalent, Inc.
Gene Delivery Technologies Market Segmentation:
By Mode
- Biological
- Adenovirus
- Retrovirus
- AAV
- Lentivirus
- Other viruses
- Non-viral
- Chemical
- Physical
By Application
- Gene Therapy
- Cell Therapy
- Vaccines
- Research
By Method
- Ex vivo
- In vivo
- In vitro
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East Africa (MEA)
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41578
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
