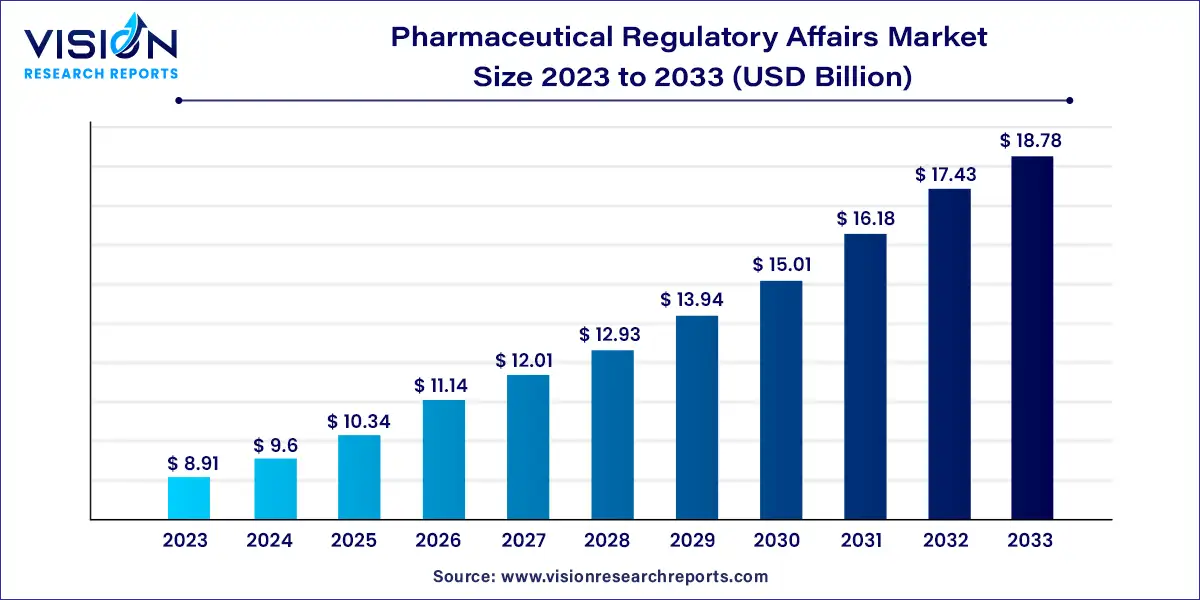
The global pharmaceutical regulatory affairs market size was surpassed at USD 8.91 billion in 2023 and is expected to hit around USD 18.78 billion by 2033, growing at a CAGR of 7.74% from 2024 to 2033.
Pharmaceutical regulatory affairs encompass the processes and activities involved in ensuring compliance with regulations set by governmental agencies regarding the development, manufacture, and distribution of pharmaceutical products. It involves liaising with regulatory bodies, conducting clinical trials, and obtaining approvals for drug marketing.
Key Pointers
- The Asia Pacific region accounted largest revenue share of 39% in 2023.
- In terms of category, the drug segment held the largest revenue share of over 59% in 2023.
- The biologics segment is anticipated to witness the fastest CAGR of 7.87% over the forecast period.
- Based on services, the regulatory writing & publishing segment dominated the market with the largest revenue share of 38% in 2023.
- Legal representation is anticipated to witness the fastest CAGR of 8.52% over the forecast period.
- The oncology segment dominated the market with the largest revenue share of 35% in 2023.
- The immunology segment is anticipated to witness the fastest CAGR of 9.29% over the forecast period.
- In terms of product stage, in 2023, the clinical studies segment held the largest market share of 48%.
Get a Sample@ https://www.visionresearchreports.com/report/sample/40775
Importance of Regulatory Affairs in Pharmaceuticals
Understanding Regulatory Affairs
Regulatory affairs professionals navigate the complex landscape of regulations to ensure that pharmaceutical companies adhere to guidelines set by regulatory authorities. They are responsible for submitting applications for drug approval, maintaining compliance throughout the product lifecycle, and addressing regulatory issues as they arise.
Regulatory Bodies and Their Roles
Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe play a crucial role in setting standards and regulations for pharmaceutical products. They assess the safety, efficacy, and quality of drugs before granting approval for market authorization.
Trends in Pharmaceutical Regulatory Affairs
Technological Advancements
Advancements in technology, such as artificial intelligence and big data analytics, are transforming the landscape of pharmaceutical regulatory affairs. These tools streamline regulatory processes, enhance data analysis, and improve decision-making, ultimately accelerating the drug development and approval process.
Global Harmonization
Global harmonization initiatives aim to standardize regulatory requirements across different regions, promoting consistency and efficiency in the regulatory process. Collaboration among regulatory authorities worldwide facilitates the timely approval of pharmaceutical products and enhances patient access to innovative treatments.
Challenges Faced in Pharmaceutical Regulatory Affairs
Compliance Issues
Maintaining compliance with evolving regulations poses a significant challenge for pharmaceutical companies. Regulatory requirements vary across regions and are subject to frequent updates, requiring organizations to adapt quickly to ensure continued compliance and avoid regulatory penalties.
Changing Regulations
The dynamic nature of regulatory frameworks presents challenges for pharmaceutical companies, as changes in regulations can impact product development timelines and market access. Keeping abreast of regulatory updates and proactively addressing compliance issues is essential for navigating regulatory complexities.
Career Opportunities in Regulatory Affairs
Skills and Qualifications Required
A career in regulatory affairs requires a combination of scientific knowledge, regulatory expertise, and communication skills. Professionals in this field typically hold degrees in pharmacy, life sciences, or a related discipline, along with specialized training in regulatory affairs.
Job Roles and Responsibilities
Roles in regulatory affairs span various functions, including regulatory submissions, quality assurance, and compliance management. Regulatory affairs professionals liaise with internal teams, regulatory authorities, and external stakeholders to ensure regulatory compliance and facilitate product approvals.
Read More: https://www.heathcareinsights.com/pruritus-therapeutics-market/
Pharmaceutical Regulatory Affairs Market Key Companies
- Freyr
- IQVIA Inc
- ICON plc
- WuXi AppTec
- Charles River Laboratories
- Labcorp Drug Development
- Parexel International Corporation
- Pharmalex GmbH
- Pharmexon
- Genpact
Pharmaceutical Regulatory Affairs Market Segmentations:
By Services
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Writing
- Publishing
- Product Registration & Clinical Trial Applications
- Other Services
By Category
- Drugs
- Innovator
- Preclinical
- Clinical
- Pre-Market Approval (PMA)
- Generics
- Preclinical
- Clinical
- Pre-Market Approval (PMA)
- Innovator
- Biologics
- Biotech
- Preclinical
- Clinical
- Pre-Market Approval (PMA)
- ATMP
- Preclinical
- Clinical
- Pre-Market Approval (PMA)
- Biosimilars
- Preclinical
- Clinical
- Pre-Market Approval (PMA)
- Biotech
By Indication
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
By Product Stage
- Preclinical
- Clinical studies
- PMA
By Service Provider
- In-house
- Outsourcing
By Company Size
- Small
- Medium
- Large
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/40775
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
