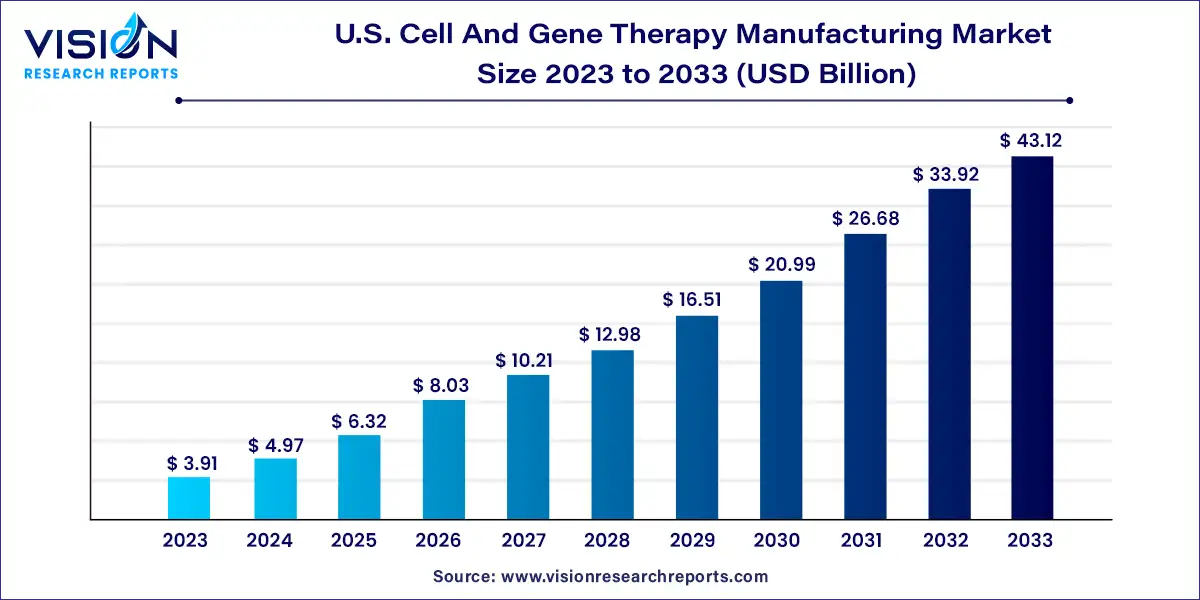
The U.S. Cell and gene therapy manufacturing market size was estimated at around USD 3.91 billion in 2023 and it is projected to hit around USD 43.12 billion by 2033, growing at a CAGR of 27.13% from 2024 to 2033.
Key Pointers
- By Therapy Type, the cell therapy manufacturing segment held the largest revenue share of 57% in 2023.
- By Therapy Type, the gene therapy segment is anticipated to grow at the noteworthy CAGR from 2024 to 2033.
- By Scale, the pre-commercial and R&D scale manufacturing generated the maximum market share of 73% in 2023.
- By Mode, the contract manufacturing segment contributed the largest market share of 66% in 2023.
- By Mode, the in-house manufacturing segment is anticipated to grow at the noteworthy CAGR during the forecast period.
- By Workflow, the process development segment held the largest revenue share of 17% in 2023.
Get a Sample@ https://www.visionresearchreports.com/report/sample/41265
Introduction to Cell and Gene Therapy
Cell and gene therapy involve the manipulation of human cells or genes to treat, prevent, or potentially cure diseases. These therapies often entail the introduction of genetic material into targeted cells, aiming to address underlying genetic defects or bolster the body’s natural defenses against illnesses.
Importance of cell and gene therapy in healthcare
The advent of cell and gene therapy marks a paradigm shift in healthcare, offering personalized treatment approaches tailored to individual patients. These therapies hold immense potential for treating genetic disorders, cancer, autoimmune diseases, and other conditions that have traditionally been challenging to manage.
U.S. Cell And Gene Therapy Manufacturing Market Top Trends:
- Advancements in Gene Editing Technologies: Technologies like CRISPR-Cas9 are revolutionizing gene editing, allowing for precise modifications to DNA sequences. These advancements enhance the efficiency and accuracy of cell and gene therapy manufacturing processes.
- Automation and Robotics: The integration of automation and robotics streamlines manufacturing workflows, reducing the risk of human error and improving scalability. Automated systems enable high-throughput production of cell and gene therapies, enhancing productivity and consistency.
- Personalized Medicine: Cell and gene therapies offer personalized treatment approaches tailored to individual patients’ genetic profiles. As our understanding of genetics and disease mechanisms grows, personalized medicine becomes increasingly feasible, driving the development of targeted therapies.
- Expanded Applications: The scope of cell and gene therapy applications continues to expand beyond traditional areas like genetic disorders and cancer. Researchers are exploring new therapeutic avenues for conditions such as neurodegenerative diseases, cardiovascular disorders, and infectious diseases.
- Investment and Funding: The U.S. cell and gene therapy manufacturing market attract significant investment and funding from both public and private sectors. Venture capital firms, pharmaceutical companies, and government agencies are pouring resources into research, development, and infrastructure to support the growth of the industry.
- Regulatory Evolution: Regulatory agencies are adapting to the unique challenges posed by cell and gene therapy manufacturing. Efforts to streamline approval processes, establish clear guidelines, and ensure product safety are essential for fostering innovation while maintaining regulatory compliance.
- Collaborative Initiatives: Collaboration among industry stakeholders, including academia, biotech companies, and regulatory bodies, accelerates innovation and knowledge sharing in the field. Consortia, partnerships, and consortiums facilitate collaborative research, precompetitive data sharing, and the development of industry standards.
- Supply Chain Optimization: Optimizing the supply chain is crucial for ensuring the timely and cost-effective delivery of cell and gene therapies to patients. Efforts to enhance logistics, storage, and distribution networks improve accessibility and affordability, addressing logistical challenges associated with personalized therapies.
By Therapy Type:
-
- Cell Therapy: This segment focuses on therapies that involve the transplantation of cells into a patient’s body to treat diseases or repair damaged tissues. Cell therapy encompasses a wide range of applications, including stem cell therapy, immune cell therapy, and regenerative medicine.
- Gene Therapy: Gene therapy involves the delivery of genetic material into a patient’s cells to correct genetic defects, modify gene expression, or enhance therapeutic responses. This segment includes both ex vivo and in vivo gene therapy approaches, targeting various genetic disorders and diseases.
By Product Type:
-
- Viral Vectors: Viral vectors serve as vehicles for delivering therapeutic genes into target cells. Adeno-associated viruses (AAVs), lentiviruses, and retroviruses are commonly used viral vectors in gene therapy manufacturing.
- Non-Viral Vectors: Non-viral vectors encompass a diverse range of delivery systems, including plasmid DNA, nanoparticles, liposomes, and electroporation-based techniques. These vectors offer advantages such as reduced immunogenicity and enhanced safety profiles.
- Cell-Based Products: Cell-based products include cell therapies derived from various cell sources, such as mesenchymal stem cells (MSCs), T cells, dendritic cells, and induced pluripotent stem cells (iPSCs). These products hold therapeutic potential for regenerative medicine, immunotherapy, and tissue engineering applications.
By Application:
-
- Oncology: Oncology represents a prominent application area for cell and gene therapies, with a focus on treating various types of cancer. Therapeutic approaches include chimeric antigen receptor (CAR) T-cell therapy, tumor-infiltrating lymphocytes (TILs), and oncolytic viruses.
- Genetic Disorders: Cell and gene therapies offer promising treatment options for genetic disorders characterized by inherited mutations or genetic abnormalities. Conditions such as hemophilia, muscular dystrophy, and cystic fibrosis are among the target indications for gene therapy interventions.
- Neurological Disorders: Neurological disorders present significant unmet medical needs, driving research into cell and gene therapy interventions. Therapeutic strategies aim to address conditions such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), and spinal cord injuries.
- Cardiovascular Disorders: Cell-based therapies hold potential for regenerating damaged cardiac tissues and restoring heart function in patients with cardiovascular disorders, including myocardial infarction, heart failure, and ischemic heart disease.
By End User:
-
- Hospitals and Clinics: Hospitals and clinics serve as primary points of care for administering cell and gene therapies to patients. These healthcare facilities provide specialized infrastructure, expertise, and supportive care services for managing complex therapeutic interventions.
- Research Institutes: Research institutes play a crucial role in advancing the development and translation of cell and gene therapy innovations from the laboratory to clinical practice. Academic research centers and biotechnology incubators contribute to preclinical studies, translational research, and clinical trials.
Read More: https://www.heathcareinsights.com/clinical-nutrition-for-cancer-care-market/
U.S. Cell And Gene Therapy Manufacturing Market Key Companies
- Lonza
- Bluebird Bio Inc.
- Catalent Inc.
- F. Hoffmann-La Roche Ltd
- Samsung Biologics
- Boehringer Ingelheim
- Cellular Therapeutics
- Hitachi Chemical Co., Ltd.
- Takara Bio Inc.
- Miltenyi Biotec
- Thermo Fisher Scientific
- Novartis AG
- Merck KGaA
- Wuxi Advanced Therapies
U.S. Cell and Gene Therapy Manufacturing Market Segmentations:
By Therapy Type
- Cell therapy manufacturing
- Stem cell therapy
- Non-stem cell therapy
- Gene therapy manufacturing
By Scale
- Pre-commercial/ R&D scale manufacturing
- Commercial scale manufacturing
By Mode
- Contract manufacturing
- In-house manufacturing
By Workflow
- Cell processing
- Cell banking
- Process development
- Fill & finish operations
- Analytical and quality testing
- Raw material testing
- Vector production
- Others
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41265
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308