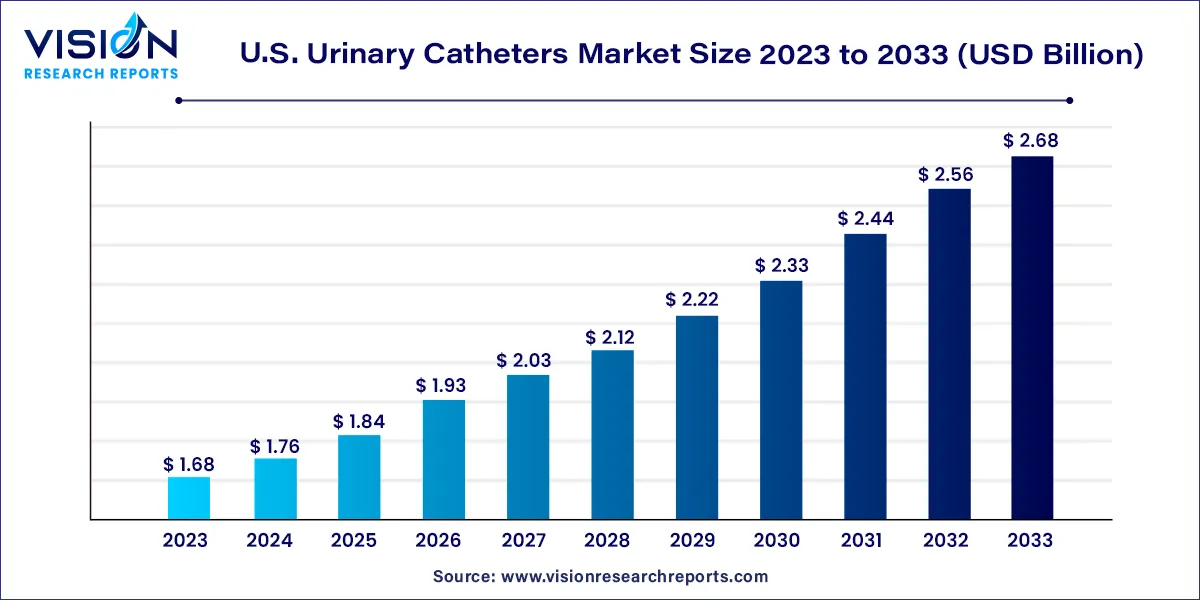
The U.S. urinary catheters market size was estimated at around USD 1.68 billion in 2023 and it is projected to hit around USD 2.68 billion by 2033, growing at a CAGR of 4.79% from 2024 to 2033.
The U.S. Urinary Catheters Market plays a crucial role in the healthcare landscape, providing essential support for patients with urinary retention or incontinence. Catheterization procedures have evolved significantly over the years, offering a range of options tailored to individual patient needs and clinical settings.
Get a Sample@ https://www.visionresearchreports.com/report/sample/41228
Types of Urinary Catheters
Indwelling Catheters
Indwelling catheters, also known as Foley catheters, are designed for continuous drainage of the bladder and are commonly used in hospitalized patients or those with impaired bladder function.
Intermittent Catheters
Intermittent catheters are used for periodic emptying of the bladder and are typically employed by individuals with spinal cord injuries or neurogenic bladder dysfunction.
External Catheters
External catheters, or condom catheters, are non-invasive devices worn externally by male patients to collect urine, offering a discreet and comfortable alternative to traditional catheterization methods.
Market Size and Growth
Current Market Statistics
The U.S. Urinary Catheters Market is characterized by steady growth, driven by increasing prevalence of urinary disorders, aging population, and advancements in catheter technology.
Projected Growth Trends
Analysts project continued expansion of the urinary catheters market, fueled by rising demand for minimally invasive urinary management solutions, growing adoption of homecare catheterization, and expanding healthcare infrastructure.
Key Players and Products
Leading Manufacturers
Key players in the U.S. urinary catheters market include Bard Medical, Hollister Incorporated, Coloplast, Teleflex, and Medtronic, among others. These manufacturers offer a diverse portfolio of catheter products catering to varying patient needs and clinical requirements.
Product Offerings
Product offerings range from standard Foley catheters and intermittent catheters to specialty catheters designed for specific patient populations, such as pediatric or geriatric patients, ensuring comprehensive coverage across healthcare settings.
Technological Innovations
Advances in Catheter Design and Materials
Technological advancements have led to the development of innovative catheter designs, materials, and coatings aimed at reducing infection risks, enhancing patient comfort, and optimizing catheter performance.
Impact on Patient Outcomes
These innovations have significant implications for patient outcomes, including reduced rates of catheter-associated urinary tract infections (CAUTIs), improved quality of life for catheter-dependent individuals, and enhanced ease of catheter insertion and removal procedures.
Regulatory Landscape
FDA Regulations and Compliance
The U.S. urinary catheters market is subject to rigorous regulatory oversight by the Food and Drug Administration (FDA), ensuring safety, efficacy, and quality standards for catheter products.
Quality Standards
Manufacturers must adhere to stringent quality standards, including ISO 13485 certification, to demonstrate compliance with regulatory requirements and ensure product reliability and consistency.
Market Drivers and Challenges
Aging Population
The aging population demographic, coupled with increasing incidence of chronic conditions such as urinary incontinence and neurogenic bladder disorders, is a primary driver of market growth.
Healthcare-associated Infections
Despite technological advancements, catheter-associated urinary tract infections remain a significant challenge, necessitating ongoing efforts to improve catheter design, infection control measures, and antimicrobial coatings.
Reimbursement Issues
Reimbursement policies and insurance coverage for urinary catheters vary, posing challenges for patients and healthcare providers in accessing and affording essential catheterization supplies.
Application in Healthcare
Hospital Settings
Urinary catheters play a vital role in hospital settings, facilitating urinary drainage, monitoring of urine output, and management of urinary retention or incontinence among hospitalized patients.
Homecare
The growing trend towards home-based healthcare has led to increased demand for homecare urinary catheterization solutions, empowering patients to manage their urinary needs independently and enhancing overall quality of life.
Read More: https://www.heathcareinsights.com/next-generation-sequencing-market/
U.S. Urinary Catheters Market Key Companies
- Hollister, Inc.
- Medtronic PLC
- Boston Scientific Corp.
- BD (C.R. Bard, Inc.)
- Cook Medical
- ConvaTec, Inc.
- Teleflex, Inc.
- Coloplast
- B. Braun Melsungen AG
- Medline Industries, Inc.
- J and M Urinary Catheters LLC
- Edwards Lifesciences Corporation
- Abbott Laboratories
U.S. Urinary Catheters Market Segmentations:
By Product Type
- Intermittent Catheters
- Foley/Indwelling Catheters
- External Catheters
By Application
- Benign Prostate Hyperplasia
- Urinary Incontinence
- Spinal Cord Injury
- Others
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41228
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
