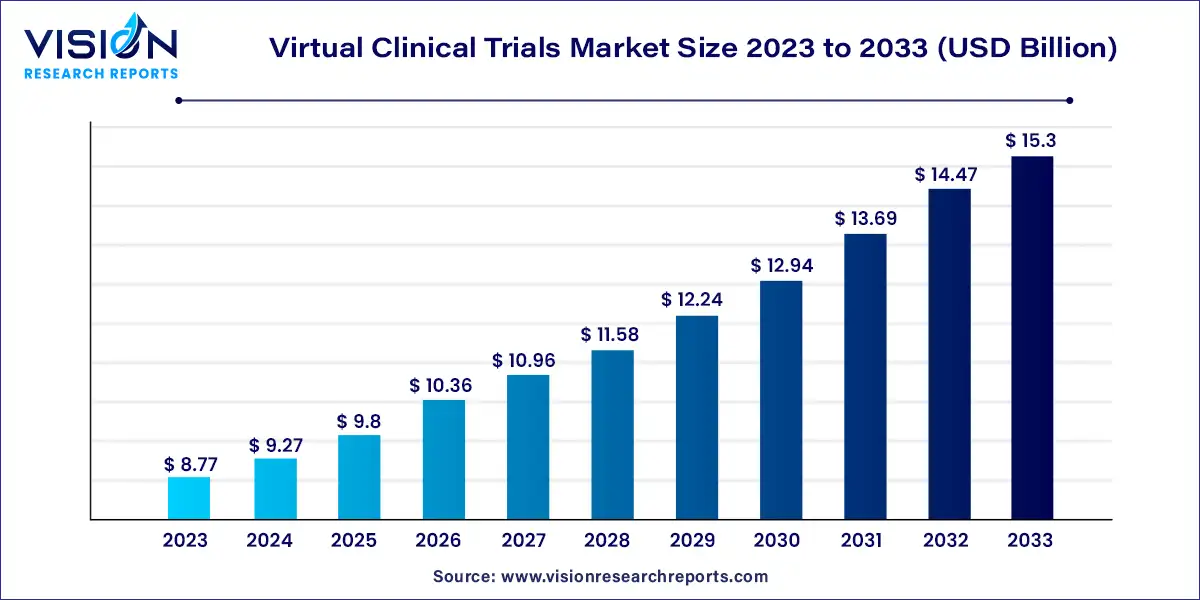
The global virtual clinical trials market size was estimated at around USD 8.77 billion in 2023 and it is projected to hit around USD 15.3 billion by 2033, growing at a CAGR of 5.72% from 2024 to 2033.
Understanding the Essence of Virtual Clinical Trials
Virtual clinical trials represent a paradigm shift in the traditional methodology of conducting clinical research. Unlike conventional trials that necessitate physical presence, virtual trials leverage digital technologies to facilitate remote participation. Through the seamless integration of telemedicine, wearable devices, and digital data collection platforms, these trials transcend geographical barriers, enhancing accessibility and inclusivity.
Virtual Clinical Trials Market Overview
Virtual clinical trials, also known as remote or decentralized trials, are a modern approach to conducting clinical research that leverages digital technologies to enable participation from subjects in their own homes or other convenient locations. Unlike traditional clinical trials that require participants to visit physical sites such as hospitals or clinics, virtual trials utilize telemedicine, wearable devices, mobile apps, and other remote monitoring tools to collect data and conduct assessments.
Get a Sample@ https://www.visionresearchreports.com/report/sample/38141
Driving Forces Behind the Growth
The exponential growth of virtual clinical trials can be attributed to a confluence of factors driving innovation and adoption within the healthcare ecosystem. Key drivers include:
- Technological Advancements
Rapid advancements in digital health technologies have catalyzed the evolution of virtual clinical trials. From remote patient monitoring to real-time data analytics, these innovations empower researchers to conduct trials with unprecedented efficiency and accuracy.
- Patient-Centric Approach
Virtual clinical trials prioritize patient convenience and comfort, offering participants the flexibility to engage from the comfort of their homes. By minimizing travel requirements and streamlining the trial process, these trials mitigate barriers to participation and enhance patient recruitment and retention.
- Cost-Efficiency
The cost-saving potential of virtual clinical trials is a compelling incentive for sponsors and stakeholders. By eliminating the need for extensive infrastructure and logistical expenses associated with traditional trials, virtual models offer a more economical alternative without compromising on scientific rigor or quality.
Key Market Trends and Opportunities
- Expansion of Therapeutic Areas
The application of virtual clinical trials extends across a diverse spectrum of therapeutic areas, ranging from chronic diseases to rare conditions. As research methodologies evolve and regulatory frameworks adapt to accommodate virtual modalities, the scope of clinical research continues to expand, unlocking new opportunities for innovation and discovery.
- Collaboration and Partnerships
Collaborative initiatives between pharmaceutical companies, technology providers, and regulatory agencies are driving the widespread adoption of virtual clinical trials. By fostering strategic partnerships and interdisciplinary collaborations, stakeholders are accelerating the development and implementation of novel trial designs and digital solutions.
- Regulatory Landscape
The evolving regulatory landscape surrounding virtual clinical trials underscores the need for proactive engagement and compliance. Regulatory agencies are increasingly receptive to innovative trial designs that prioritize patient safety and data integrity, paving the way for expedited approvals and market access.
Read More: https://www.heathcareinsights.com/active-pharmaceutical-ingredients-market/
Virtual Clinical Trials Market Key Companies
- ICON, plc
- Parexel International Corporation
- IQVIA
- Covance
- PRA Health Sciences
- LEO Innovation Lab
- Medidata
- Oracle
- CRF Health
- Clinical Ink
- Medable, Inc.
- Signant Health
- Halo Health Systems
- Croprime
Virtual Clinical Trials Market Segmentations:
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- CNS
- Autoimmune/Inflammation
- Cardiovascular Disease
- Metabolic/Endocrinology
- Infectious Disease
- Oncology
- Genitourinary
- Ophthalmology
- Others
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/38141
You can place an order or ask any questions, please feel free to contact sales@visionresearchreports.com| +1 650-460-3308
